Abstract
Menin inhibition has antileukemia activity in both mixed-lineage leukemia-rearranged (MLL-r) and nucleophosmin (NPM1)-mutated (NPM1c) AML by suppressing the menin-dependent HOX/MEIS gene signature pre-clinically and clinically. However, co-mutations are common in patients with MLL-r or NPM1c AML and menin inhibition in combination with other targeted agents has potential to enhance efficacy. FLT3 is frequently mutated in MLL-r and NPM1c AML. We previously reported that menin inhibition by SNDX-50469 (SNDX) synergized with BCL-2 inhibition by venetoclax (VEN) in vitro using primary AML patient samples and in vivo in a patient-derived xenograft (PDX) model of NPM1/FLT3-ITD/FLT3-TKD mutated AML. However, cells surviving the SNDX and VEN combination at the end of treatment had increased p-FLT3 signaling (Carter BZ et al., Blood 2021). We therefore investigated whether FLT3 inhibition with gilteritinib (Gil) could enhance the efficacy of co-targeting menin and BCL-2 using the same PDX model harboring NPM1/FLT3-ITD/-TKD mutations.
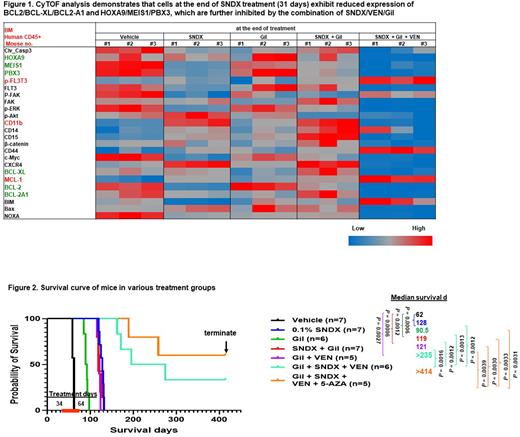
We treated PDX-bearing NSG mice with vehicle control, SNDX, Gil, SNDX + Gil, VEN + Gil, or SNDX + Gil + VEN. CyTOF analysis of bone marrow (BM) leukemia cells at the end of treatment demonstrated that the SNDX + Gil + VEN combination targeted leukemia stem/progenitor cells more effectively than SNDX, Gil, or SNDX + Gil. Furthermore, SNDX markedly reduced BCL-2, BCL-XL, BCL2-A1, MEIS1, and PBX3 proteins invivo, which were further reduced by the combination of SNDX, Gil, and VEN (Fig. 1). All treatment arms suppressed leukemia burden in peripheral blood, spleen, and BM and significantly extended survival (Fig. 2) compared to vehicle-treated controls (median 62 days). SNDX (128 days) was significantly more effective than Gil (90.5 days; P = 0.0001). SNDX + Gil (119 days) did not further improve survival compared to SNDX alone, possibly because the two agents have overlapping effects on FLT3 signaling: Gil inhibits FLT3 activity, while menin inhibition suppresses FLT3 levels. The survival duration of the Gil + VEN (121 days) and SNDX + Gil groups did not differ. Even with the reduced Gil (25 vs. 35 mg/kg) and VEN (35 vs. 50 mg/kg) doses compared to the single agent or two-drug combination groups, the SNDX + Gil + VEN combination extended survival (>235 days), significantly longer than SNDX, Gil, SNDX + Gil, or VEN + Gil. Survival in the 3-drug combination group was much longer than in the SNDX + VEN group (143 days), that we previously reported (Carter BZ et al., Blood 2021).
To determine if hypomethylating agents further improve survival, we also treated mice with the SNDX + Gil + VEN + 5-Azacytidine (5-AZA) combination. The median survival duration had not been reached when the experiment was terminated on day-414. Importantly, 2/6 mice in the triple- and 3/5 mice in the quadruple-drug treatment groups lived close to the life expectancy of normal NSG mice with no detectable leukemia cells in spleens and BMs when the experiment was terminated on day-414, suggesting that these combinations potentially eliminated all leukemia cells resulting in cures.
Conclusions: Findings demonstrate that the combined inhibition of menin, BCL-2, and FLT3 has profound activity against AML and AML stem progenitor cells with NPM1/FLT3-ITD/-TKD mutations. The combination reduces the HOX/MEIS signature and the antiapoptotic BCL-2 proteins, resulting in major survival benefit. The quadruple combination of SNDX + Gil + VEN + 5-AZA resulted in cure of >50% of mice carrying triple mutant AML. Results warrant the clinical development of concomitant inhibition of menin, BCL-2, and FLT3 combined with a hypomethylating agent in NPM1/FLT3-mutant AML.
Disclosures
Carter:PinotBio: Research Funding; Syndax: Research Funding; Revolution Medicines: Research Funding. Ordentlich:Syndax: Current Employment. Andreeff:Brooklyn ITX: Research Funding; Glycomimetics: Consultancy; Medicxi: Consultancy; Cancer UK: Membership on an entity's Board of Directors or advisory committees; NCI: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding; Kintor Pharmaceutical: Research Funding; Syndax: Consultancy, Research Funding; Breast Cancer Research Foundation: Research Funding; Aptose: Consultancy, Membership on an entity's Board of Directors or advisory committees; Senti Bio: Consultancy, Research Funding; Pinot Bio: Research Funding; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo Inc.: Consultancy, Research Funding; Oxford Biomedical UK: Research Funding; Oncolyze: Current holder of stock options in a privately-held company; Chimerix: Current holder of stock options in a privately-held company; Reata: Current holder of stock options in a privately-held company; German Research Council: Membership on an entity's Board of Directors or advisory committees; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal